


The ETERNAL Horizon Europe Research and Innovation Action has just made seven industrial case study summaries publically available. The easily-read two-page documents provide a concise summary of the key features, progress to date, and further steps involved as several of the project's applied research efforts start to yield fruit in an at or near industrial ready state.
08 September 2025
This video describes how concerted efforts in ETERNAL's case studies distributed across a range of industrial use cases and the extended value chain for pharmaceuticals are making a real and positive impact upon the future sustainability and competitiveness of the sector.
05 September 2025
The latest scientific paper produced by researchers in the ETERNAL project emphasises the potential role of enzymes in supporting the transition towards a more sustainable future pharmaceutical industry.
04 September 2025
We are delighted to announce that the 6th International Conference on Risk Assessment of Pharmaceuticals in the Environment (ICRAPHE), being held in Aveiro, Portugal, from October 20 – 21 2025, will this year feature a special conference session entitled ‘Risk Assessment for Impact Reduction across the Pharmaceuticals Life Cycle’ dedicated to results emerging from the ETERNAL project.
31 August 2025
This newsletter, the sixth since the ETERNAL project started, provides ample opportunities to dig deeper into both the project’s research illuminating the environmental risks surrounding pharmaceutical manufacture and positive responses from the industry to minimize the risks through technological innovation and standardization for less impactful, more efficient, sustainable and safe by design processes.
15 August 2025
Two new CEN Workshops are being planned which will complement the activities of the EU Research project ETERNAL. These activities will seek to establish standardized methods for 'determining the encapsulation efficiency of RNA and DNA in LNPs using fluorometry' and 'early-stage sustainability assessment for chemical and biochemical manufacturing processes' respectively.
15 May 2025
Congratulations to researchers at ETERNAL industrial partner AstraZeneca whose recent publication on recovery and reuse of homogeneous palladium catalysts via organic solvent nanofiltration has just been named as a 2025 HOT article by the editorial and review team at RSC Green Chemistry.
04 April 2025
Two scientific posters on the industrial case studies which they are leading in ETERNAL were presented by Angelini Pharma in the context of a dissemination event for the LIFE-GREENAPI project hosted at its Fine Chemicals plant in Aprilia. Find out more about optimized use of solvents in API manufacture and the use of twin screw extrusion processing in pharmaceutical development.
31 March 2025
ETERNAL is proud to be a contributing organisation at the 2025 edition of the UK and Ireland’s premier event covering all aspects of the pharmaceutical supply chain, Making Pharmaceuticals, taking place at the Ricoh Arena, Coventry, UK on 29th and 30th April 2025.
12 March 2025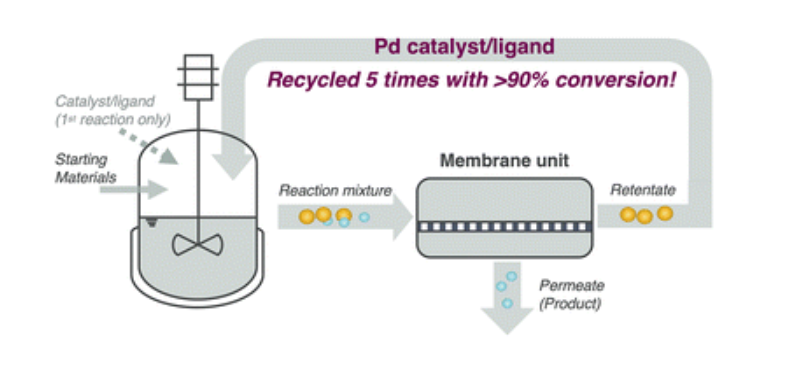
A new open-access, peer-reviewed paper by ETERNAL indiustrial partner AstraZeneca demonstrates the effective recovery of homogeneous palladium catalysts from reaction mixtures using commercial organic solvent nanofiltration (OSN) membranes. The paper presents a real pharmaceutical manufacturing case study to synthesize AZD4625, without altering the existing catalyst/ligand system. The recovered catalyst and ligand were successfully reused up to five times, maintaining over 90% conversion. Life cycle assessment shows that the sustainability of the process could be further enhanced by using greener bio-derived solvents and implementing solvent recovery to reduce solvent consumption.
28 February 2025
The first annual meeting of the group of EU funded projects on Green Pharmaceuticals was held on 10th December 2024 in Brussels. Hosted by the European Commission, representatives of the ETERNAL project and our four sister projects currently within the cluster provided updates on the projects' objectives and progress, and had productive discussions with a range of stakeholders from across the Community.
11 December 2024
The ETERNAL project consortium's 5th General Assembly meeting in Barcelona on 24th and 25th October 2024 heard reports of continuing strong progress as the project's six industrial case study teams start to transition the focus of their work towards scale up for impact.
28 October 2024
The ETERNAL project recently contributed to the success of a well-received Speciality Chemicals Symposium run by the Royal Society of Chemistry in conjunction with the Chemspec Europe 2024 exhibition in Düsseldorf, Germany.
18 July 2024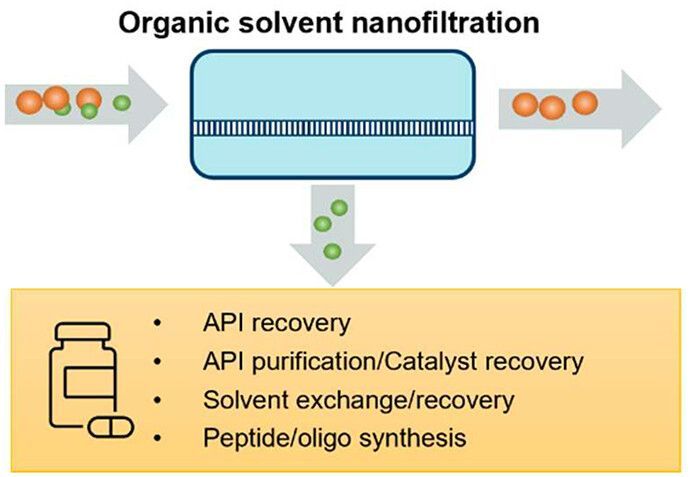
Separation and purification in organic solvents are indispensable procedures in pharmaceutical manufacturing. Organic solvent nanofiltration (OSN) offers a low energy, low waste generating, and easily scalable alternative to the conventional method sof distillation or chromatography. A newly open-access published review from ETERNAL partners AstraZeneca comprehensively summarizes the recent progress of organic solvent nanofiltration and its applications in the pharmaceutical industry.
22 March 2024
We look forward into the year ahead with a sense of growing excitement as the ETERNAL project’s collaborative research and case study strands gather pace. With several parts of the project’s work programme starting to deliver early results and work progressing strongly across the board, we are looking forward to sharing some of the early highlights and our latest thinking at two notable gatherings of industrialists and researchers from the pharmaceutical and specialty chemicals sectors respectively in the first half of 2024.
08 February 2024
Meet the people working in ETERNAL to translate the alignment of ETERNAL’s goals and AstraZeneca’s sustainability strategy into demonstrable developments in the use of membranes in pharmaceutical processing to recover and purify solvents with energy efficiency, remove impurities, and reduce ecotoxicity of waste streams.
22 September 2023This site uses cookies that enable us to make improvements, provide relevant content, and for analytics purposes. For more details, see our Cookie Policy. By clicking Accept, you consent to our use of cookies.