


A newly released public deliverable of the ETERNAL project lays out the key-drivers and a possible regulatory roadmap for “compliant-by-design” strategies applying the concepts of “greener design” to be delivered in Europe.
01 December 2023
A key aspect of evaluating the environmental impact and sustainability of pharmaceuticals is assessing the risk that they may pose to ecosystems. A new public report from the ETERNAL project provides an overview of the current EMA regulation covering the environmental risk assessment of pharmaceuticals, followed by a comparison against the new proposed Regulation and Directive published earlier in 2023. The report concludes with a series of recommendations for integrating the latest scientific knowledge into pharmaceutical risk assessment to help fill gaps in the current and proposed legislation.
01 December 2023
The application of digital technologies to the pharmaceutical industries has the potential to significantly increase the agility, efficiency and flexibility, the sustainability of the drug manufacturing processes, and the quality of the medicines produced. Despite all this, the pharmaceutical industry has been more hesitant to adopt digital technologies than other industries due to the complexities and expertise required in its development and manufacturing processes. However, with the growing demand for traditional and new drugs, there is a clear need for digitalization in the sector.
01 June 2023
Using standards as a knowledge source in the earliest possible stages of research and innovation avoids duplication of work and provides the basis for future marketable products. This deliverable identifies and describes the standardisation technical committees (TCs) at European and International level related to the ETERNAL project as well as the published standards (existing and under development) that could be relevant and useful for all the project activities.
01 March 2023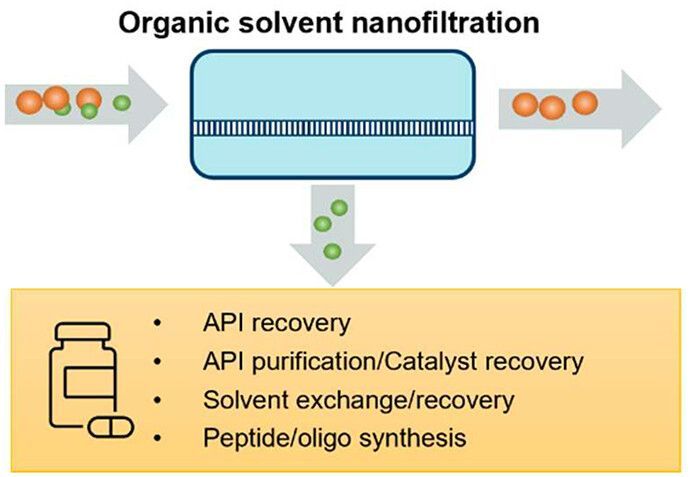
Separation and purification in organic solvents are indispensable procedures in pharmaceutical manufacturing. Organic solvent nanofiltration (OSN) offers a low energy, low waste generating, and easily scalable alternative to the conventional method sof distillation or chromatography. A newly open-access published review from ETERNAL partners AstraZeneca comprehensively summarizes the recent progress of organic solvent nanofiltration and its applications in the pharmaceutical industry.
22 March 2024
Artificial Intelligence (AI) and Big Data (BD) presents an unprecedented opportunity within the framework outlined by the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) to enhance quality assurance, streamline drug development processes, increase traceability at supply chain level, and ultimately improve quality and safety of medicinal products. A new report generated by the ETERNAL Research and Innovation Action discusses the ethical considerations and legislative imperatives for integrating AI and BD within the ICH guidelines to ensure responsible usage and sustainable business outcomes.
07 June 2024This site uses cookies that enable us to make improvements, provide relevant content, and for analytics purposes. For more details, see our Cookie Policy. By clicking Accept, you consent to our use of cookies.