

Ongoing access to safe, high quality and effective pharmaceutical treatments for citizens and animals is a vital part of fulfilled, equitable living. Amongst many things, the COVID-19 pandemic has amplified the need for sustainable pharmaceutical supply chains and consumption patterns. At the same time, we must avoid any undue impact of pharmaceutical residues on the environment. Indeed, our health and well-being strongly depend on a healthy environment.
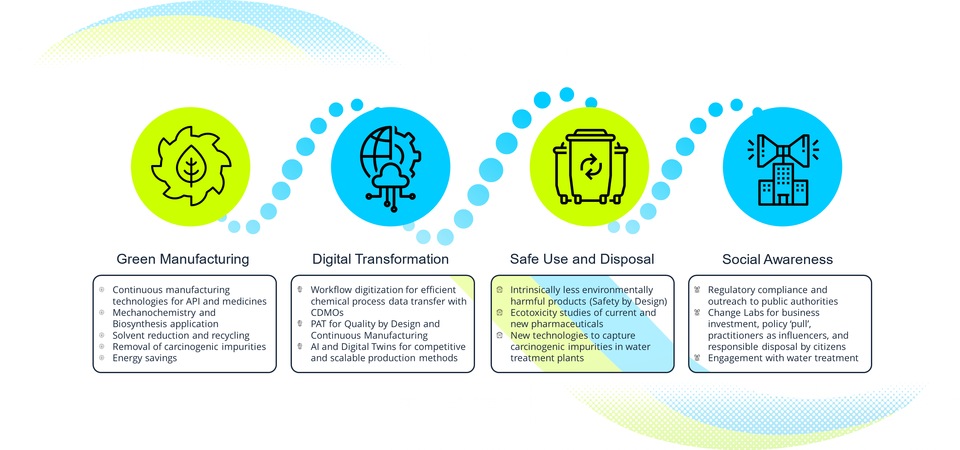
ETERNAL’s overall purpose is to contribute to sustainable development of pharmaceutical manufacture, use and disposal, by using and promoting full life cycle approaches covering design, manufacture, use, and disposal. This includes assessing the environmental risks of active pharmaceutical ingredients, residues, metabolites, and other chemicals and by-products of the production process. The project team forms an industry-research-compliance partnership intent on delivering upon these goals by:
Co-designing application-industry oriented R&D and scale-up in six industrial case studies. Focused upon the key enabling technologies of Green Chemistry, Mechanochemistry, and Digitalization, these variously encompass
reduced use of solvents
application of greener solvents;
optimized solvent recycle/recovery options for processes with potentially carcinogenic impurities like nitrosamines
application of mechanochemistry using Holt Melt Extrusion processing in the production of pharmaceutical products
more eco-efficient purification/capture routes for solvents and wastewater with biobased products
innovative workflow digitalization, Process Analytical Technology (PAT) and Digital Twin solutions to enable Quality by Design and Continuous Manufacturing for competitive and scalable methods of production
Assessing the regulatory implications of adopting the innovations to ensure a pathway to compliance
Generating new scientific knowledge on the environmental fate and eco-toxicological effects of pharmaceuticals
Catalyzing behavioural change, participation and social innovation for reducing the environmental impacts of pharmaceuticals in terms of safe use and disposal.
 Project Partners
Project PartnersThe ETERNAL consortium unites a world class consortium of sixteen organisations from seven countries across Europe. The team brings together complementary knowledge and expertise from academic and specialist research institutes, leading businesses in the pharmaceutical industry, and innovative SME businesses in whole process design, technology/digitalization, environmental engineering, innovation services, and international scientific and regulatory affairs.
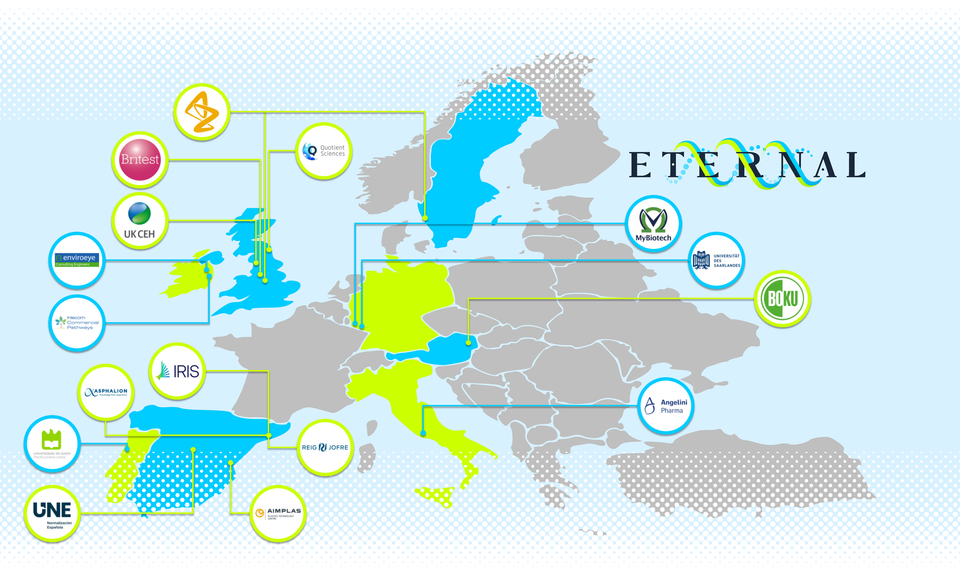
The ETERNAL consortium has strong linkages in research and innovation with many other national, European and international projects. We are keen to build new relations and collaborations with other relevant projects with the view to maximizing synergies and establishing enduring international scientific relationships.
This site uses cookies that enable us to make improvements, provide relevant content, and for analytics purposes. For more details, see our Cookie Policy. By clicking Accept, you consent to our use of cookies.