


With the project entering its fourth and final year, the news has been welcomed by project co-ordinator, Pablo Ferrer Pérez, Project Manager at Spanish RTO AIMPLAS:
“We are delighted to bring such great news about the strong progress of ETERNAL’s case studies to as wide an audience as possible, and will be looking forward to telling all our stakeholders more in the coming months as we look to broaden the impact of our efforts.”
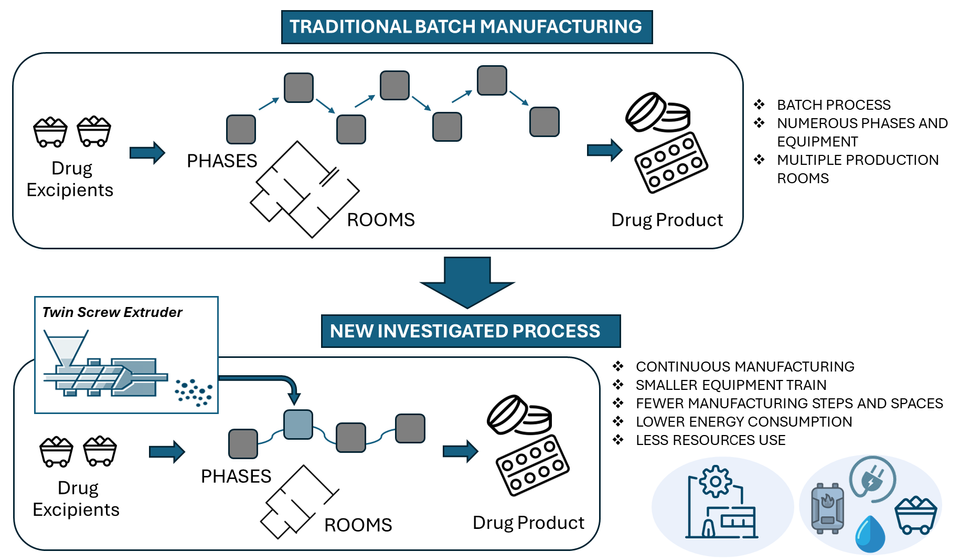
Angelini Pharma has used Twin Screw Granulation successfully to produce granules for immediate-release tablets with fewer steps and less materials, avoiding water addition and energy-intensive phases, as a potential alternative to the current batch manufacturing process.
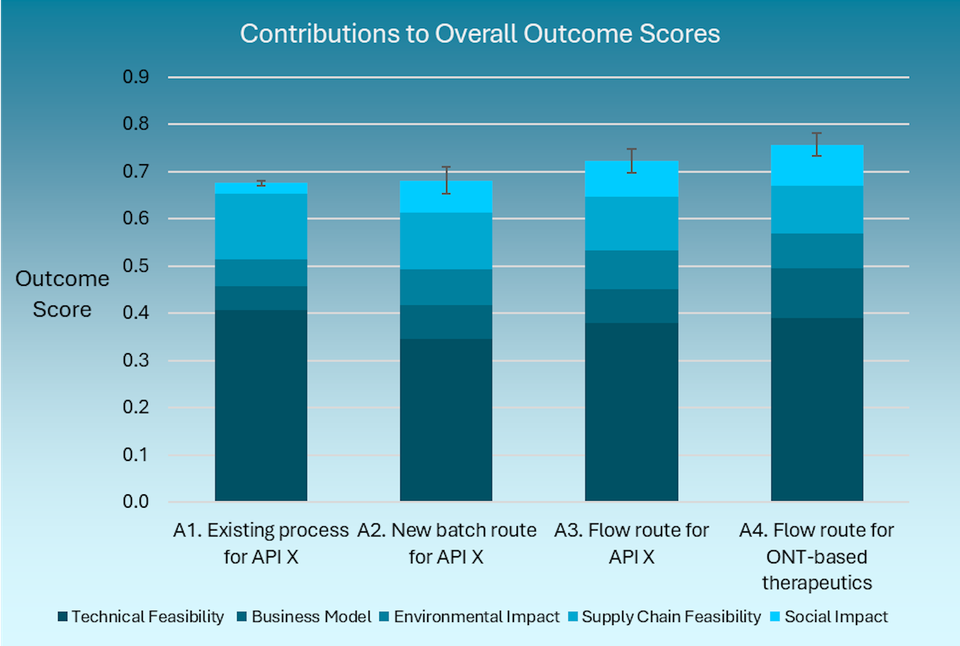
Britest has developed a framework that teams can use to think systematically about the many criteria which go into deciding whether a proposed innovation enhances sustainability or not, coupling it with an improved method for multiple-criteria decision analysis in the presence of uncertainty. The combination can be used to guide a facilitated decision-making process towards clear, documented outcomes.
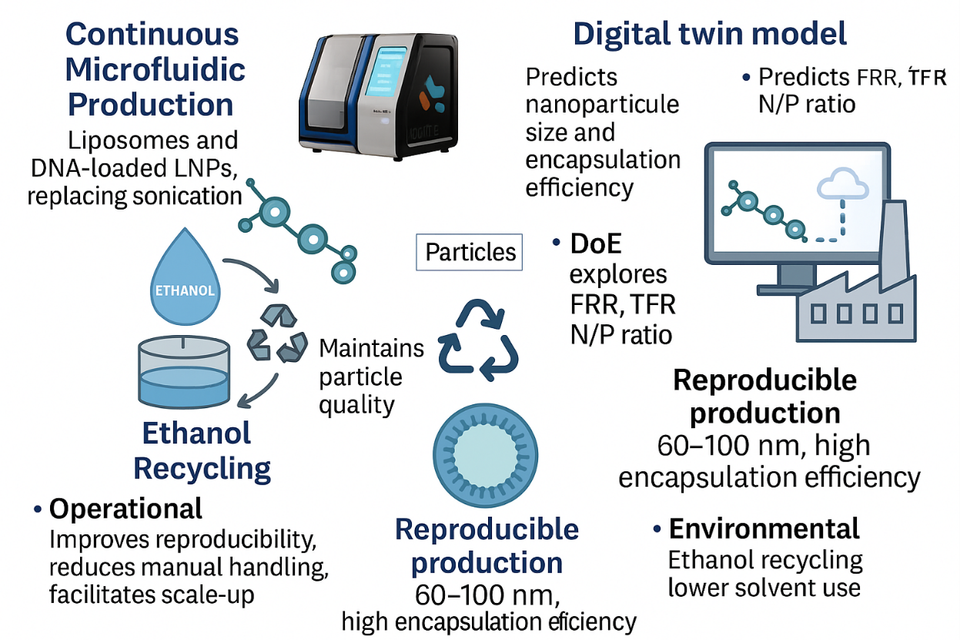
Laboratorio Reig Jofre has introduced a continuous microfluidic platform to produce both diclofenac-loaded liposomes and DNA-loaded lipid nanoparticles (DNA-LNP). Design of experiments (DoE) combined with digital twin simulation of particle properties, enables predictive formulation, reducing the need for physical experimentation.
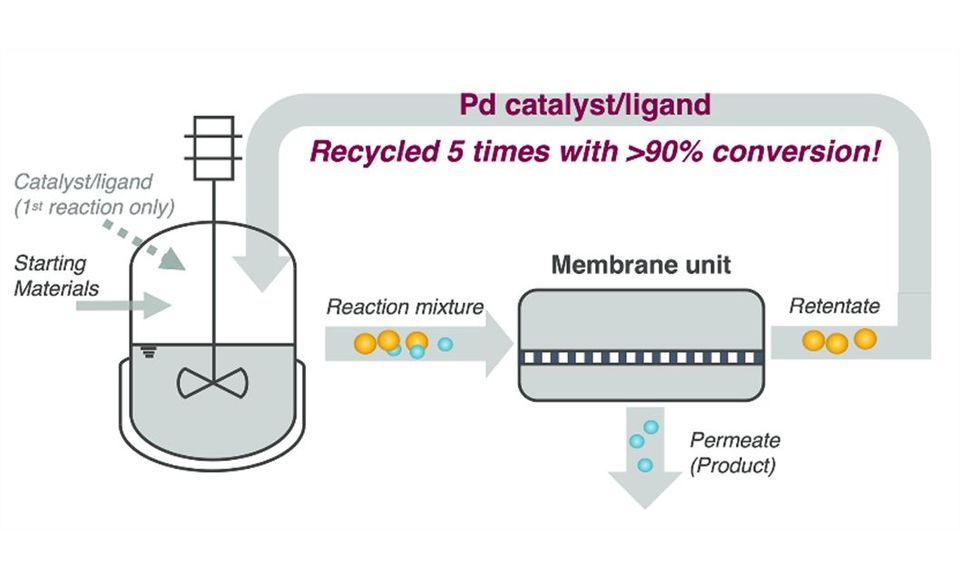
AstraZeneca has developed a process using organic solvent nanofiltration (OSN) membrane technology to efficiently recover and reuse palladium catalysts during the synthesis of an important oncology drug. This minimizes energy consumption and waste production, promoting a more sustainable approach to catalyst management in pharmaceutical manufacturing.
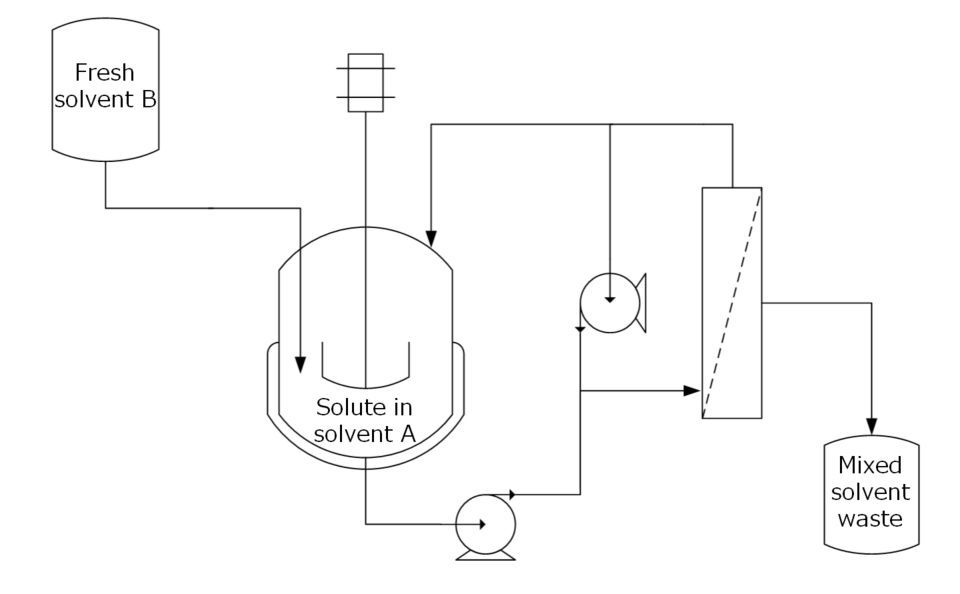
Organic solvent nanofiltration (OSN) offers an energy-efficient alternative to distillation, using membrane technology to achieve low temperature, low energy solvent exchange. Coupled with inline Fourier Transform infrared spectroscopy (FTIR), solvent composition can be monitored dynamically, enhancing the overall process efficiency.
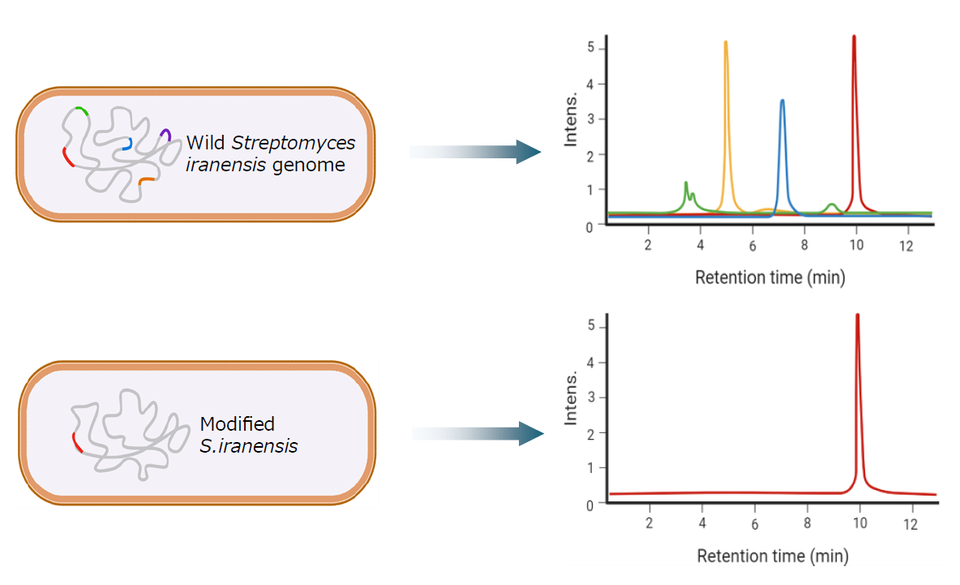
Many microbial producer strains produce multiple by-products alongside their target compound. Preventing the expression of these by-products opens the way to safer, more stream-lined and sustainable pharmaceutical bio-manufacturing processes. A simple and straightforward genome editing platform for bacteria has been used to precisely delete targeted DNA regions in a producer bacterium used for making the immunosuppressant drug rapamycin. The method is potentially adaptable to any microbial system.
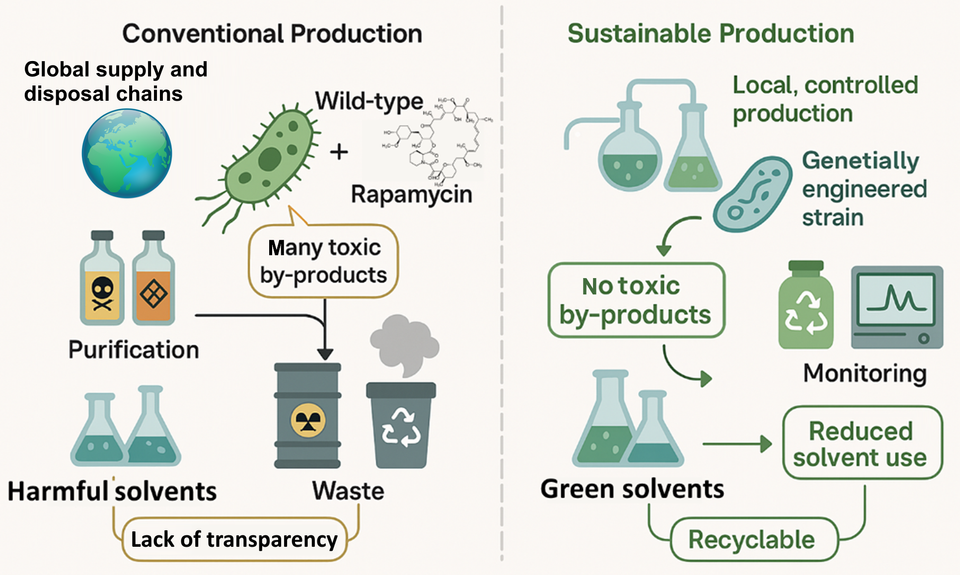
Large quantities of environmentally harmful chemicals are generally required to purify active ingredients produced by bio-process based routes. MyBiotech GmbH has developed an innovative continuous end-to-end process which reduces the carbon footprint and the environmental impact of rapamycin production assessed by the toxicity of the process chemistry and products.
This site uses cookies that enable us to make improvements, provide relevant content, and for analytics purposes. For more details, see our Cookie Policy. By clicking Accept, you consent to our use of cookies.