


Hosted jointly by close neighbours IRIS Technology Solutions and Laboratorio Reig Jofre along with the project’s third partner with a Barcelona office Asphalion, the meeting provided a valuable opportunity for updates from the project’s work stream leaders and dialogue between researchers drawn from across the project’s twelve-strong consortium.
As ETERNAL moves through the mid-point of its four-year programme, the project’s six focal point industrial case studies are moving forwards strongly with their mission to demonstrate a range of concepts in greener by design, more sustainable process and product innovation for pharmaceutical development and manufacture. Researchers at Italian industrial partner Angelini Pharma for example are currently working with colleagues at Spanish technology centre AIMPLAS to optimize the energy efficiency and output characteristics (e.g. particle size distribution) of a successfully demonstrated continuous twin-screw melt granulation approach for the manufacture of a solid tablet form product, using the mechanical energy input to the process materials as a reliable scale-up factor. In a second case study, Angelini have also produced some promising preliminary results towards the complete upgrade in capability of the distillation used to achieve a challenging mixed solvent separation and recovery duty associated with a current commercial process, and have been studying the opportunity to substitute green, bio-based solvent alternatives for conventional solvents in drug substance manufacture whilst work continues.
Laboratorio Reig Jofre have made excellent headway with their research into the better use of solvents through microfluidic based manufacturing of various liposome encapsulated payloads from conventional chemical-based drugs to oligonucleotides and mRNA formulations. This work is being guided in part (in particular around changes in lipid formulation) by virtual Design of Experiments using a Digital Twin developed by Spanish advanced process and quality control specialists IRIS. More conventional real-world Design of Experiments also has a big part to play in helping elucidate and harness the relationship between key process control parameters and the output liposome particle size and encapsulation efficiency. In this case study, some promising options have also been identified for the treatment of residual active found in process aqueous waste solutions. Both solid adsorbents (activated carbon) and mixed matrix membranes have been shown to deliver in excess of 90% removal of the target compound from aqueous solution.
Effective recovery of value-bearing streams and mitigation of environmental risk from process residues also feature strongly in the case study being led by AstraZeneca’s researchers in the UK and Sweden. Their investigations into the potential use of membrane diafiltration to attain the recovery and reuse of precious-metal based homoegenous catalysts on the one hand, and to perform the ubiquitously important task of solvent-exchange in a more time and energy efficient fashion on the other has been reported recently.
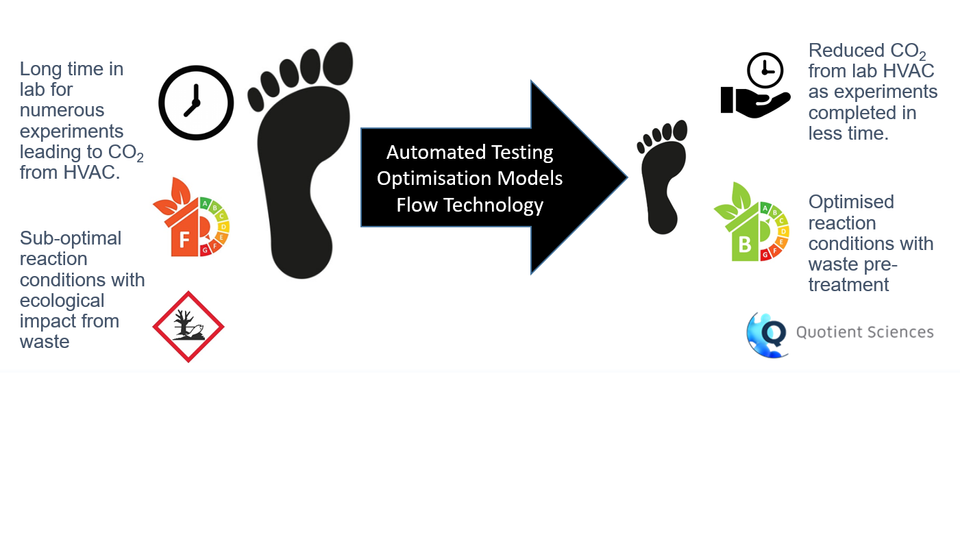
Greener-by-Design Synthesis Methods: the use of characterisation, modelling, automation and flow technology as part of a methodology to rapidly get to optimised API synthesis conditions that have a minimised environmental footprint.
UK-based CDMO & CRO Quotient Sciences is thinking differently about how manufacturing process development can be done in ways that work for the environment and for their customers. They are bringing together a combination of high fidelity and agent based process modelling, integrated development lab-scale continuous synthesis engineering (both CSTR cascade and pure plug flow), and advanced process monitoring and control systems to deliver upon their vision for an accelerated pathway for new development projects. Put simply, development time equates to money and energy, and so the shorter the time to a successful process development, the greater the economic and environmental benefits. With the processes characterised and the models developed and validated, carefully choosing the right environmental optimization factor to target becomes a key part of a successful. sustainable development. In the second half of the project Quotient intend to demonstrate these Greener-by-Design Synthesis Methods in an industrially relevant environment (TRL6) through application of the methodology to client projects, to the installation and operational qualification of equipment, and to the synthesis of demonstration batches.
Finally, the joint efforts of pharmaceutical biotechnologists at the University of Saarland and the scientists and engineers at innovative CDMO MyBiotech GmbH are leading to the development of a platform for decreasing toxic and bioactive side products during fermentative production of pharmaceuticals and green downstreaming processing using continuous technologies. By developing a genome-engineered version of the relevant producer bacterial strain, Saarland’s researchers seek to optimize and target bio-synthetic reactions exclusively towards the target molecule, avoiding the formation of undesired compounds which tends to occur with wild strain types. Currently they have achieved the first three and are progerssing with the fourth in a targeted sequence of ten deletions from the producer strain genome. Meanwhile, MyBiotech have taken forward the use of the first of the engineered strains produced at Saarland to successfully implement biomass cultivation on a 10 litre scale, paving the way for downstream processing development and optimisation.
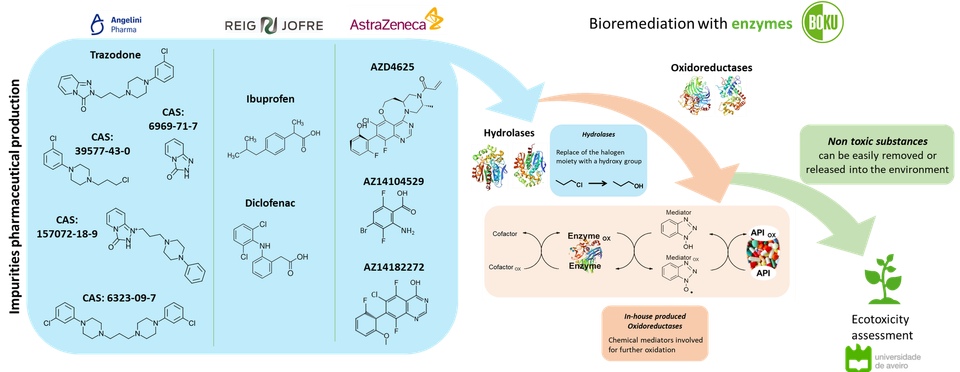
ETERNAL’s industrial case study work forms the heart of the project’s work to advance the technology readiness of innovative, green technologies for future sustainability in the EU pharmaceuticals supply chain, but it is not the whole picture. Elsewhere, complementary work into the remediation of pharmaceutical manufacturing waste streams by either enzymatic or photocatalytic means is being pursued by BOKU (the University of Natural Resources and Life Sciences, Vienna) and AIMPLAS respectively.
Here researchers have been targeting the oxidative destruction of both actives and by-product materials associated with several of the case study processes, in BOKU’s case developing the concept of a multi-enzymatic cascade treatent approach. In such a way, potentially harmful or ecotoxic materials can be broken down into non-toxic substances that can easily be removed or released to the environment without any adverse effects. A poster describing some preliminary results of using the cascade approach to bioremediation to compounds of interst to several of the ETERNAL case studies (see summary below) was recently awarded a best poster prize at the 10th IUPAC International Conference on Green Chemistry, held in Beijing, China.
Congratulations to lead author Filippo Fabri and his colleagues for this recognition for their work!
This site uses cookies that enable us to make improvements, provide relevant content, and for analytics purposes. For more details, see our Cookie Policy. By clicking Accept, you consent to our use of cookies.